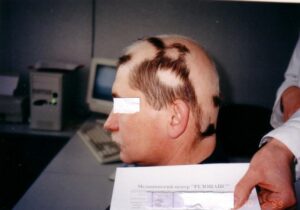
 Effective treatment for hair loss has eluded physicians and scientists for generations, regardless of the type of hair loss or the mechanism underlying it. Alopecia areata is an autoimmune form of hair loss where hair is rapidly lost from the scalp, eyelashes, and eyebrows. The treatment options for the condition have been limited, but clinical trials of a new drug, baricitinib, have proven promising for adults suffering from alopecia areata.
Effective treatment for hair loss has eluded physicians and scientists for generations, regardless of the type of hair loss or the mechanism underlying it. Alopecia areata is an autoimmune form of hair loss where hair is rapidly lost from the scalp, eyelashes, and eyebrows. The treatment options for the condition have been limited, but clinical trials of a new drug, baricitinib, have proven promising for adults suffering from alopecia areata.
Lilly, the pharmaceutical company that has developed baricitinib, has published results from their phase 3 trials on the efficacy and safety of the drug in adults with the hair loss condition in the New England Journal of Medicine. Their two randomized, placebo-controlled trials, referred to as BRAVE-AA1 and BRAVE AA-2 investigated the impact of the drug on adults with severe alopecia areata, defined as a score of 50 or higher when the Severity of Alopecia Tool (SALT) was applied.
BRAVE-AA1 included 654 participants, and BRAVE-AA2 included 546. Patients received either 4 mg of the drug each day, 2 mg of the drug each day, or placebo. They were tested at week 36 with SALT.
Data from the trials showed that the baricitinib treatment was superior to placebo in terms of its associated with hair regrowth after 9 months of treatment. The higher dosage appeared to be more effective than the lower dose. However, there were some side effects associated with baricitinib, including acne, elevated creatine kinase levels, and elevated low and high-density lipoprotein cholesterol levels.
While these results appear promising in terms of the ability of this new therapy to provide hair regrowth for adults suffering from alopecia areata, the authors of the study conclude that longer trials are required for a better understanding of both the safety and efficacy of baricitinib in this hair loss condition.
Reference
King B, Ohyama M, Kwon O, et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. New England Journal of Medicine. Published online March 26, 2022. doi:10.1056/NEJMOA2110343
